2021.02.26 Patent
Criteria for “Advantageous Effect” in Determination of Inventive Step are Clarified
Overview
It is useful to deepen your understanding of how an advantageous effect is considered in determination of inventive step in the Japanese practice.
A recent decision of the supreme court regarding an invention of use of a medical compound (Judgement of the Supreme Court of Japan, 3rd Petty Bench, dated August 27, 2019 on Case No. 2018 (Gyo-Hi) 69) clarified the criteria for an advantageous effect in determination of inventive step.
As shown in Fig. 1 below, the decision clarified that, in determination of inventive step of an invention of Compound A for example, whether Compound A renders an “unexpected remarkable effect” should be determined based on whether the effect of Compound A is “unexpected” from and “remarkable” for the structure of Compound A, and not directly based on whether another compound (Compound B) having an effect comparable to the effect of Compound A existed as of the reference date for determining inventive step.
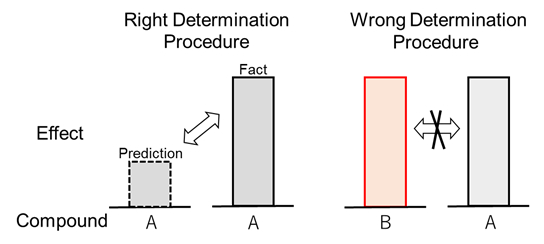
Fig.1 Determination of Unexpected Remarkable Effect
(According to Supreme Court Decision)
(According to Supreme Court Decision)
In the field of pharmaceuticals, it is effective to assert that a claimed compound renders an “unexpected remarkable effect” when arguing inventive step of an invention of a compound in the prosecution. However, in the previous Japanese practice, we have come across some cases that were denied of inventive step of an invention of a compound in the prosecution because of an existence of other compound having the same or comparable effect albeit having a different structure.
Now that the criteria for an advantageous effect in determination of inventive step is clarified by this decision of the Supreme Court, it will have a great significance in strategy formulation in the prosecution of patent applications in the field of pharmaceuticals.
We present below some detailed information you may find useful in arguing an advantageous effect with respect to determination of inventive step and also in strategy formulation in the prosecution for your patent applications.
1. Determination of Inventive Step
We begin with a brief explanation of procedures in determining inventive step in the Japanese practice as a basis of considering advantageous effects.)
According to the examination guidelines, an examiner determines inventive step of a claimed invention by assessing whether he/she can reason that a person skilled in the art could have easily achieved the claimed invention from a primary cited invention.
This reasoning by the examiner is shown in Fig. 2 below. The examiner comprehensively evaluates each of “Factors in support of the non-existence of inventive step” and “Factors in support of the existence of inventive step” shown in Fig. 2 to determine inventive step.
In this reasoning, if an advantageous effect compared to the cited invention is found to be beyond predictions based on the state of the art in the technical field, it is evaluated as a factor in support of the existence of inventive step.
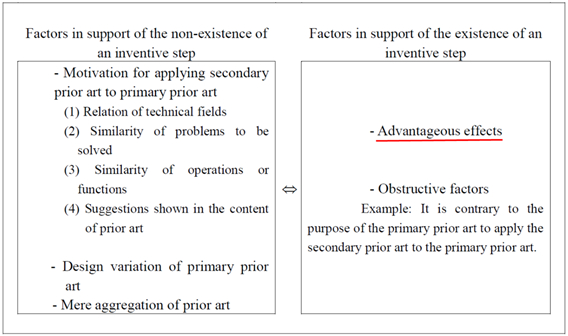
Fig.2 Major Factors Used in Reasoning
(Cited from Examination Guidelines)
(Cited from Examination Guidelines)
2. How to Determine Unexpected Remarkable Effect
There have been three theories as described below in terms of how to determine an unexpected remarkable effect.
<Theory I> Comparison with Primary Cited Invention
A theory that an unexpected remarkable effect should be an effect of the claimed invention that is remarkable and unexpected as compared only with an effect of the Primary cited invention.
<Theory II> Comparison with Claimed Invention
A theory that an unexpected remarkable effect should be an effect of the claimed invention that is remarkable and unexpected as compared with an effect that a person skilled in the art can predict from the structure of the claimed compound (as of the reference date for determining inventive step).
<Theory III> Comparison with State of the Art
A theory that an unexpected remarkable effect should be an effect of the claimed invention that is remarkable and unexpected as compared only with comparable effects that have been achieved by the state of the art in the technical field as of the reference date for determining inventive step (including those effects rendered by inventions having different structures from the claimed invention).
The decision of the Supreme Court this time seems to be made based on Theory II, Comparison with Claimed Invention. Therefore, this decision clarified that, for example, a remarkableness of an advantageous effect of a claimed invention of a compound should be determined based on a comparison with an effect that is predictable from the structure of the claimed compound, and that the remarkableness of the advantageous effect should not be denied based on a fact that the same or a comparable effect was already known to be rendered by “other compound” having a structure different from the claimed compound as of the reference date for determining inventive step.
3. Practical Guide
If an examiner denies an advantageous effect of a claimed compound based on an existence of other compound and its effect in an office action, the applicant is able to refute the determination of the examiner.
If a claimed new compound renders an effect that is advantageous compared with those effects that are conceivable based on the structure of the claimed compound, there is a possibility of obtaining a patent. This can be an advantage, for example, in the prosecution of a patent application for a new medicine that is developed to achieve a certain known effect with a compound having a structure different from other known compounds.
4. Conclusion
The decision of the Supreme Court we introduced above is the first decision that states the criteria of an advantageous effect, and thus will be published as reference information in the Examination Handbook, which is used as a supplemental material of the examination guidelines.
We will continue to pay close attention to those prosecutions that are similar to this Supreme Court case.
Edited by Yusuke Tsuji and Kenshi Takenaka

